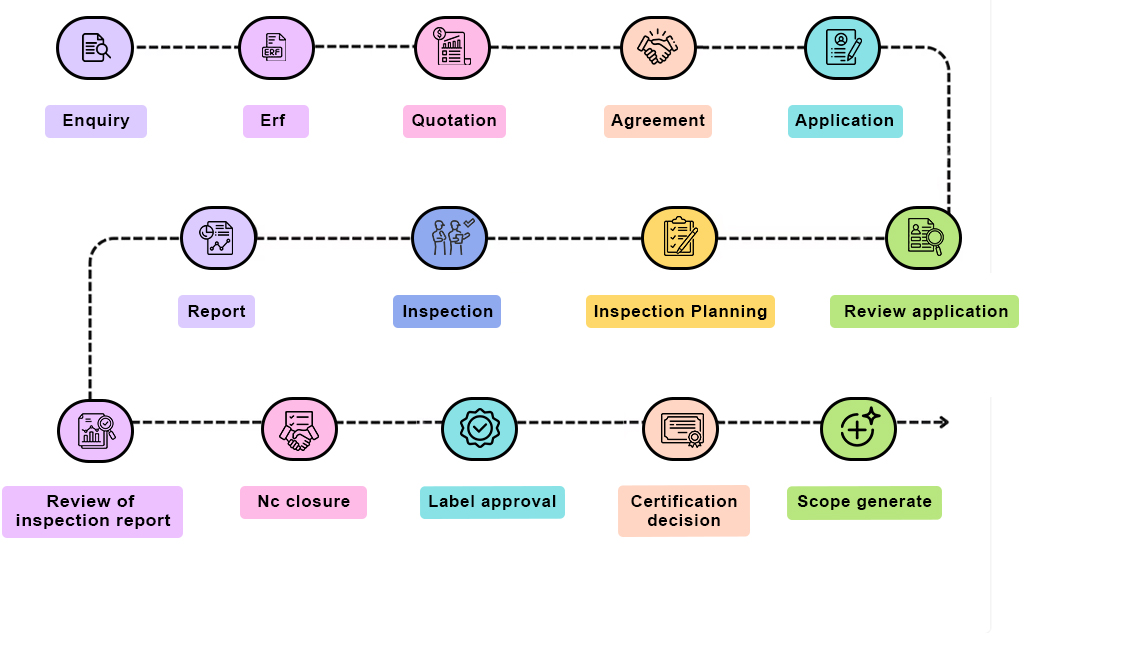
=== PROCESSING UNIT CERTIFICATION ===
PROCESSING UNIT CERTIFICATION
To maintain the quality and integrity of the organic
product, any
handling and processing factory or unit should be
certified, and this
certification can be obtained under Processing unit
certification.
Important points to be considered for the certification
are processing
and handling of organic products should be done
separately in time or
place from handling and processing of non organic
products.
Pollution sources shall be identified, and contamination
avoided.
Processing methods should be based on mechanized,
physical and
biological processes. The vital quality of an organic
ingredient shall
be maintained throughout each step of its
processing.
DOCUMENT
NOTE : The purpose of this list is to make the operator aware about the certification requirements and documentation. The documents mentioned in the list given below shall be maintained by the operator and be submitted to the Certification Body at the time of inspection.
- Company registration
- Organization chart
- Application & CV’s of the staff
- Appointment orders of the staff
- Conflict of interest & confidentiality
- Annual training schedule of the staff
- Staff training record
- Subcontract if any
- Processing area/ unit map
- Label drafts
- List of machineries with capacity
- SOPs for all products
- Machine log book
- Purchase record
- Inward register
- Processing record
- Sales record
- Outward register
- Product balance table
- Complaints register
- Input guarantee of raw/ purchased material
- List of ingredients/food additives used
- Non GMO declaration for purchased inputs
- Cleaning schedule
- Cleaning register
- Pest control record for processing unit & Storages
PROCEDURE